Mitochondrial replenishment for the restoration of mitochondrial function in age-related diseases
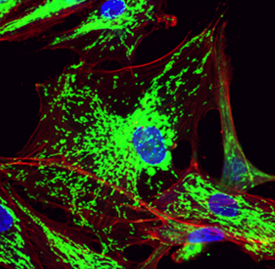
Mitochondrial dysfunction is a hallmark of diseases that include cancer, atherosclerosis, Alzheimer’s disease, and age-related macular degeneration (AMD). The major research focus of the lab is aimed at understanding mechanisms of mitochondrial dysfunction in the pathogenesis and progression of age-related diseases and the development of treatments that restore mitochondrial function. We demonstrated that transfer of mitochondria to breast cancer and cardiovascular cells profoundly impacted cellular respiration, mediating a shift towards improved oxidative phosphorylation. Mitochondrial transplantation to cells (including macrophages, fibroblasts) also led to decreased oxidative stress and re-balancing of mitochondrial dynamics, abrogating disease-associated processes. Our research focus has evolved from the delivery of exogenous mitochondria to the stimulation of innate mitochondrial biogenesis. In our most recent publication (Lee et al., Aging Cell 2025), mitochondrial biogenesis restored mitochondrial function and prevented senescence in cells undergoing oxidative stress. In another recent publication (Massaro et al., Neurotherapeutics 2025), mitochondrial biogenesis in neuronal cells exposed to amyloid beta (Aβ), restored mitochondrial function, reducing oxidative stress and neurotoxicity. Our laboratory continues to explore strategies aimed at restoring mitochondrial function in metabolically compromised cells.
Local and systemic, nanoparticle-based drug and gene delivery
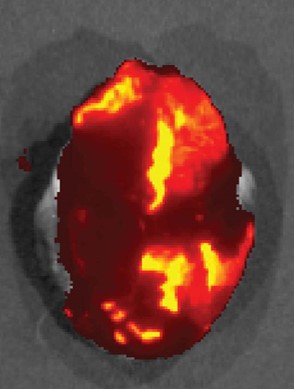
Fenestrated vasculature contributes immensely to heightened accumulation of NPs in tumors through the enhanced permeability and retention (EPR) effect. Importantly, “leaky” vasculature is present in other diseases. Hearts undergoing heart failure have endothelial dysfunction, and we demonstrated heightened particle accumulation in mice undergoing heart failure (Eur J Heart Fail, 2016, 18, 2). Similarly, endothelial remodeling exists in pulmonary arterial hypertension (PAH) and we demonstrated increased NP accumulation in a preclinical model of PAH (Int J Pharm, 2017, 524, 1-2).
Short-term pharmacokinetics limit the efficacy of cardiac pharmacotherapies. We adapted the dry pericardial puncture to access the pericardial sac for nanoparticle (NP)-based drug delivery to the heart and were one of the first labs to demonstrate the potential of exploiting the pericardial space for NP-based drug delivery to the heart (J Control Release 2017, 262). We showed long-term presence of NPs in the heart following pericardial delivery, and extensive intramural penetration and maintenance of released payload, converting the pericardial space into a local drug delivery depot to the heart and addressing the limitations of pericardial drug delivery and other local delivery strategies.
Nanoparticle-based drug and gene delivery remains an active area of research in the laboratory, particularly aimed at restoration of mitochondrial function.
Nano-inspired, MR-visible interventional devices for iCMR
Many cardiovascular interventions, including stent deployment and balloon angioplasty, are currently performed under x-ray guidance. This modality is associated with poor soft tissue contrast, the inability to properly navigate endovascular tools, and significant safety concerns in patients due to exposure to ionizing radiation and iodinated contrast agents. Magnetic resonance imaging (MRI) is a safe modality that offers high-resolution anatomic and physiologic information, and interventional CMR (iCMR) has the potential to allow for more accurate navigation and positioning of catheter-based devices, all in a radiation-free environment. However, the promise of iCMR has not translated into a clinical reality due to the scarcity of MR-compatible devices. Thus, our objective is to create application-specific, iCMR-compatible devices, and demonstrate the potential of iCMR by performing cardiovascular catheterization procedures using these devices. Work in this area was recently published in the journal Biomedical Microdevices (2019, 20, 2).