Metabolic regulation through the use of ‘biological nanoparticles’
Dysregulated mitochondrial energy transfer drives numerous diseases including cancer and heart failure. Mitochondrial transplantation is an innovative strategy that can restore favorable metabolic phenotypes in cells with aberrant energy metabolism. My laboratory functionalized isolated mitochondria with a biocompatible polymer to enhance cellular delivery and transplantation. We demonstrated that grafting of a polymer conjugate composed of dextran and triphenylphosphonium (TPP) onto isolated mitochondria protected the organelles and facilitated cellular internalization compared to uncoated mitochondria. Importantly, mitochondrial transplantation into cancer and cardiovascular cells profoundly impacted respiration, mediating a significantly enhanced shift towards improved oxidative phosphorylation. Our findings represent the first demonstration of polymer functionalization of isolated mitochondria, highlighting a viable strategy for enabling clinical applications of mitochondrial transplantation in a variety of diseases. This line of research represents our current focus area and has resulted in a publication in Advanced Science (2018, 5, 3).
Intrapericardial nanotherapeutics for sustained drug delivery to the myocardium
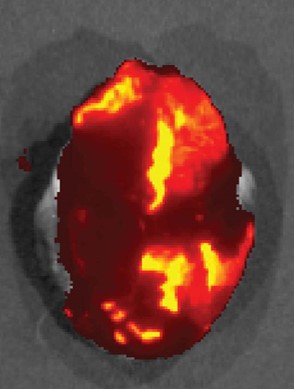
Short-term pharmacokinetics limit the efficacy of cardiac pharmacotherapies. Catheter-based approaches for local drug delivery to the heart have been developed, including intracoronary and intramyocardial administration. However, these suffer from inconsistent drug delivery, poor local retention of injectate over time, insufficient distribution to the myocardium. We adapted the dry pericardial puncture to access the pericardial sac for nanoparticle (NP)-based drug delivery to the heart. We are the first to demonstrate the potential of exploiting the pericardial space for NP-based drug delivery to the heart. We showed long-term presence of NPs in the heart following pericardial delivery, and extensive intramural penetration and maintenance of released payload, converting the pericardial space into a local drug delivery depot to the heart and addressing the limitations of pericardial drug delivery and other local delivery strategies. We are currently using this approach to deliver the anti-arrhythmic drugs and small interfering RNAs (siRNAs), with findings highlighting sustained anti-arrhythmic effects. Our research in this area has resulted in a publication in J Control Release (2017, 262).
Nanoparticle-based drug and gene delivery for heart failure and chronic lung diseases
Fenestrated vasculature from ongoing angiogenesis contributes immensely to the heightened accumulation of NPs in tumors, through a phenomenon known as the enhanced permeability and retention (EPR) effect. Importantly, this characteristic of tumor vasculature is present in other diseases. Hearts undergoing heart failure have endothelial dysfunction. We demonstrated particle accumulation in a murine model of preclinical heart failure (Eur J Heart Fail, 2016, 18, 2). Particles accumulated to a greater extent in failing hearts compared to normal hearts after intravenous (IV) administration.
We hypothesized that a similar phenomenon would occur in lungs exhibiting pulmonary arterial hypertension (PAH). Endothelial remodeling in pulmonary arterial hypertension (PAH) can induce vascular fenestrations, which were expected to facilitate NP extravasation and accumulation through disrupted endothelium. We demonstrated heightened accumulation of NPs administered systemically (IV) in a preclinical model of PAH (Int J Pharm, 2017, 524, 1-2). We examined the efficacy of NPs containing rapamycin (RAP), an mTOR inhibitor, in a monocrotaline (MCT)-induced rat model of PAH. When compared to free RAP, RAP NPs led to a much greater increase in RAP concentration in lungs from MCT-exposed rats. RAP NPs prevented PAH, displaying a marked improvement over non-treated controls.
Nanoparticle-based drug and gene delivery remains an active area of research in the laboratory, with various projects focused on pulmonary delivery for chronic lung diseases such as PAH and idiopathic pulmonary fibrosis (IPF), as well as heart failure.
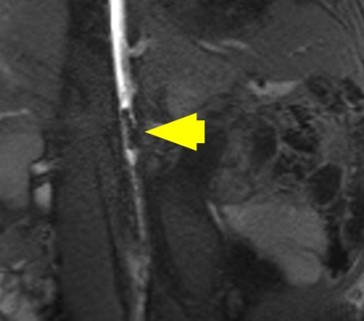
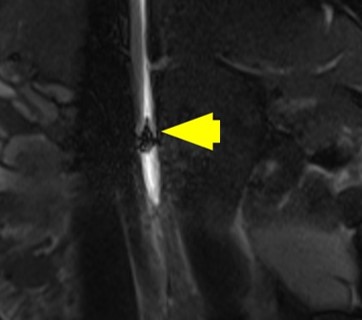
Nano-inspired, MR-visible interventional devices for iCMR
Many cardiovascular interventions, including stent deployment and balloon angioplasty, are currently performed under x-ray guidance. This modality is associated with poor soft tissue contrast, the inability to properly navigate endovascular tools, and significant safety concerns in patients due to exposure to ionizing radiation and iodinated contrast agents. Magnetic resonance imaging (MRI) is a safe modality that offers high-resolution anatomic and physiologic information, and interventional CMR (iCMR) has the potential to allow for more accurate navigation and positioning of catheter-based devices, all in a radiation-free environment. However, the promise of iCMR has not translated into a clinical reality due to the scarcity of MR-compatible devices. Thus, our objective is to create application-specific, iCMR-compatible devices, and demonstrate the potential of iCMR by performing cardiovascular catheterization procedures using these devices. Work in this area was recently published in the journal Biomedical Microdevices (2019, 20, 2).